Institute for Environmental and Gender-Specific Medicine
Kazuhisa Iwabuchi, PhD
- Professor, Laboratory for Biochemistry, Juntendo University School of Health Care and Nursing
- Associate Professor, Institute for Environmental and Gender-specific Medicine, Juntendo University Graduate School of Medicine
Iwabuchi Research Group
Members:
| Kazuhisa Iwabuchi | Professor Infection Control Nursing, Graduate School of Health Care and Nursing Laboratory for Biochemistry, Juntendo University School of Health Care and Nursing |
 |
| Hitoshi Nakayama | Associate Professor Laboratory for Biochemistry, Juntendo University School of Health Care and Nursing |
 |
| Madoka Kage | Post-Doctoral fellow | |
| Noriko Yokoyama | Researcher | |
| Kumiko Ishii | Research Assistant | |
We focus on organization and functions of glycosphingolipid-supramolecular domains in innate immunity.
Research Description:
Molecular mechanisms of glycolipid-mediated innate immunityResearch interest:
Project 1: Organization and signal transduction mechanisms of glycosphingolipid- enriched lipid raft (supramolecular domains)
Glycosphingolipids (GSL) are expressed on the outer layer of plasma membranes, and participate in transducing signals from outside to inside cells. More than 400 different oligosaccharide chains associated with GSL have been identified to date, with the number of GSL molecular species being at least 10-fold higher, due to the heterogeneity of their ceramide moieties. Several studies have demonstrated that the molecular varieties and expression patterns of GSL reflect their biological functions. In mammalian cells, ceramides are synthesized by a family of six enzymes, ceramide synthase (CerS) 1–6, each of which uses a restricted subset of fatty acyl-CoAs to N-acylate the sphingoid long chain base. The levels of expression of each CerS-encoding gene differ among tissues, suggesting that molecular variations and expression patterns of fatty acid chains of GSLs reflect the functions of these cells. GSLs cluster to form lipid rafts, which have been implicated in a number of important membrane events. However, the molecular mechanisms by which GSLs mediate cell functions remain unclear. One major issue is the association of GSLs with signal transducer molecules localized to the cytosolic side of the plasma membrane. We have addressed this issue using human neutrophilic lineage cells. Lactosylceramide (LacCer) are highly expressed on the plasma membranes of human neutrophils and macrophges. The transition temperature of LacCer is around 70oC, indicating that LacCer, under physiological conditions, is not present as a soluble single molecule in the cytosol of these cells. Indeed, exogenously added LacCer introduced into the plasma membranes of neutrophils was not detected in their cytosol. LacCer forms Lyn and/or Gαi coupled lipid rafts, which mediate neutrophil chemotaxis, phagocytosis and superoxide generation. The main types of LacCer in plasma membranes contain the very long C24 fatty acid chains. In contrast, over 70% of LacCer on plasma-membranes of neutrophilic differentiated human promyelocytic leukemia HL-60 cells (D-HL-60 cells) consists of C16:0-LacCer, with only 13.6% being C24-LacCer. D-HL-60 cells loaded with C24-LacCer showed LacCer-mediated functional activity and allowed Lyn co-immunoprecipitation by anti-LacCer antibody. Lyn knockdown by siRNA completely abolished the effect of C24-LacCer loading on the LacCer mediated functions of D-HL-60 cells. Experiments using azide-photoactivatable tritium-labeled C24- and C18-LacCer showed that C24- but not C16-LacCer was directly associated with Lyn and Gαi. These results confirm a specific direct interaction between C24-LacCer and the signal transduction molecules Lyn and Gαi, which associate with the cytoplasmic layer via palmitic chains. Therefore, LacCer species with very long fatty acids are thought to be indispensable for Lyn-coupled LacCer-enriched lipid raft-mediated neutrophil functions. Importantly, this is the one of the several mechanisms responsible for the signal transduction through glycolipid-enriched lipid rafts. Depending on physicochemical properties of glycolipids and/or components in the domains, glycolipids mediate outside-in signaling via several different mechanisms.
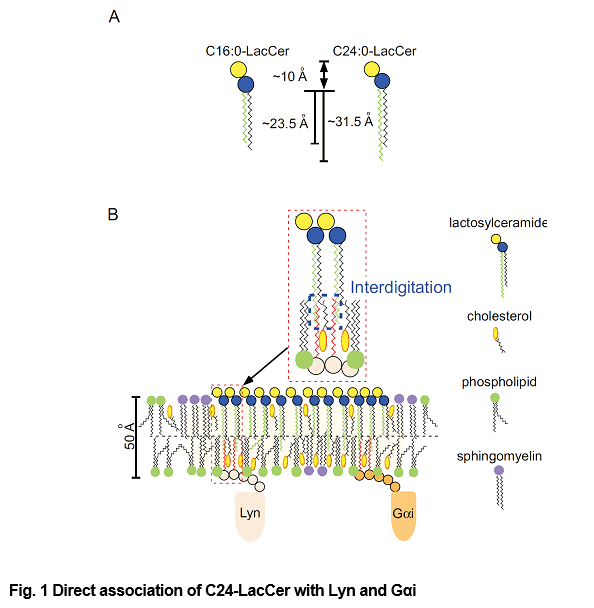
- The size of the LacCer containing C16:0 and C24:0 fatty acid chain.
- LacCer forms lipid rafts on plasma membrane of human neutrophils and acts as a pattern recognition receptor. The C24 fatty acid chains of LacCer interdigitate into inner leaflet of plasma membrane and directly interact with Lyn and Gαi to allow association of these molecules with LacCer to mediate signaling from outside to inside, resulting in neutrophil chemotaxis, migration and phagocytosis.
Project 2: Lactosylceramide-enriched lipid rafts mediated engulfment of pathogenic microorganisms by phagocytes and escape mechanisms of pathogenic mycobacteria
The innate immune system is one of the most important host defenses against invading microorganisms, including bacteria, fungi, and viruses. Professional phagocytes, such as neutrophils and macrophages, are the primary cells involved in innate immune responses. These responses are initiated by interactions between PAMPs expressed on microorganisms and PRRs on host cells. Several types of PRR, including toll-like receptors (TLRs), C-type lectin receptors (CLRs), and some GSLs, can directly sense PAMPs without opsonization by C3bi or IgG. The binding avidities of microorganisms to several types of GSL suggest that GSL-enriched lipid rafts are involved in host–pathogen interactions. Among GSLs, LacCer has been demonstrated to bind to several types of microorganisms, including Escherichia coli, Bordetella pertussis, Bacillus dysenteriae, Propionibacterium freudenreichii, and Candida albicans, suggesting the importance of LacCer in interactions between these microorganisms and host cells. In human neutrophils, we demonstrated LacCer-enriched lipid rafts acted as a PRR, resulting in several immunological functions, such as migration, phagocytosis and superoxide generation. In contrast, mouse neutrophils express only a small amount of LacCer on their cell surfaces. However, LacCer-enriched lipid raft is involved in αMβ2 integrin-dependent phagocytosis of non-opsonized microorganisms in mouse neutrophils. There are several important differences between mouse and human innate immune systems; e.g., differences in expression patterns of the Toll-like receptor family and function of the C-type lectin receptor DC-SIGN. The molecular mechanisms involved in the innate immune system differ between humans and other animals. We should always pay attention to these differences for understanding the biological functions of human phagocytes.
αMβ2 Integrin (Mac-1, CD11b/CD18) is essential for neutrophil adhesion and phagocytosis. We clearly demonstrated that αMβ2 integrin collaborated with LacCer-enriched lipid rafts during neutrophil engulfment of non-opsonized microorganisms. Although this integrin is devoid of catalytic activities responsible for signaling inside the cells, ligand binding to αMβ2 integrin delivered Src family kinase-dependent outside-in signals, followed by neutrophil activation. The αM subunit has a unique structure, containing not only a binding site for ligands but also a spatially separated carbohydrate binding domain. The latter domain binds to microorganisms-derived several carbohydrates, such as β-glucan which is a major component of fungal cell walls. Binding of β-glucan to the αM subunit can directly induce conformational changes and activate this subunit, leading to superoxide generation. We demonstrated that αMβ2 integrin-dependent outside-in signaling was mediated by Lyn molecules in LacCer-enriched lipid rafts. It is likely that the LacCer-enriched lipid rafts work as signal transduction platform for the outside-in signaling by αMβ2 integrin under non-opsonized conditions. (J Leuk. Biol. 83: 728-41, 2008)
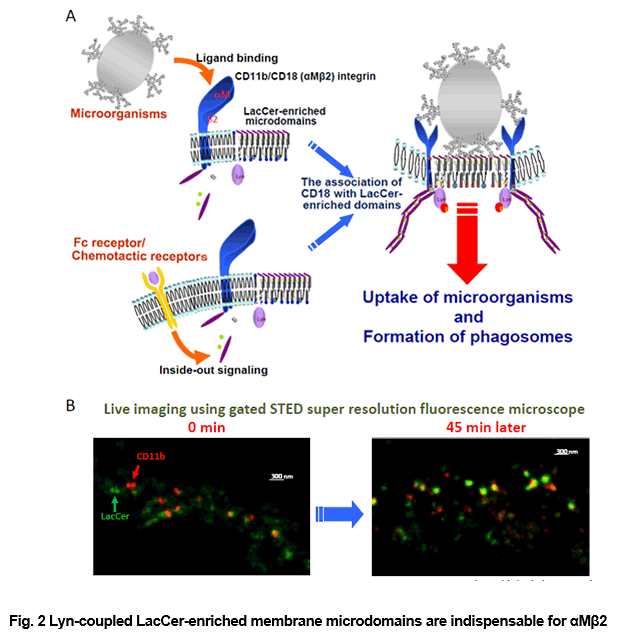
integrin-mediated phagocytosis. (A) αMβ2 integrin is associated with LacCer-enriched membrane microdomains through β2 subunit, and this subunit mediates Lyn phosphorylation. Along with the binding of PAMPs expressed on microorganisms to αMβ2 integrin, the integrin molecules are activated and undergoes a conformational change. Then these integrin molecules translocates into LacCer-enriched membrane microdomains and activates Lyn molecules. These signaling cascades lead to phagosome formation. (B) Live images of αM integrin subunit (CD11b) and LacCer using Gated STED Super resolution fluorescence microscope. Along with the incubations, CD11b went into the LacCer-enriched domains.
※ Pathogenic mycobacteria escape from the killing by human neutrophils by the interactions between mannan-capped lipoarabinomannan and LacCer
Pathogenic Mycobacterium species such as M. tuberculosis and M. avium complex (MAC) are responsible for a wide variety of human ailments. In phagocytes, pathogenic mycobacteria inhibit the fusion between lysosomal granules and their phagosomes, leading to the proposal that these properties favor their survival in phagocytes. Neutrophils have been also demonstrated to phagocytose mycobacteria without fusion of lysosomes (Azurophil granules) with phagosomes. Neutrophils play a defensive role during mycobacterial infections. Moreover, several studies have been demonstrated that neutrophil phagocytosis of M. tuberculosis results in necrosis rather than apoptosis of these cells, and that M. tuberculosis is able to escape from killing by neutrophils. It has been demonstrated that lipid rafts play some roles in engulfment of mycobacteria. We observed that neutrophils phagocytosed mycobacteria, regardless of their pathogenicity, through the binding of LacCer-enriched lipid rafts to the mannan core region of lipoarabinomannan (LAM), and that association between LacCer-enriched lipid rafts and Hck is essential for the survival of pathogenic mycobacteria in human neutrophils (Figs. 3 and 4). We are studying about the molecular mechanisms of phagocytosis of mycobacteria and escape mechanisms of pathogenic mycobacteria from killing by human phagocytes.
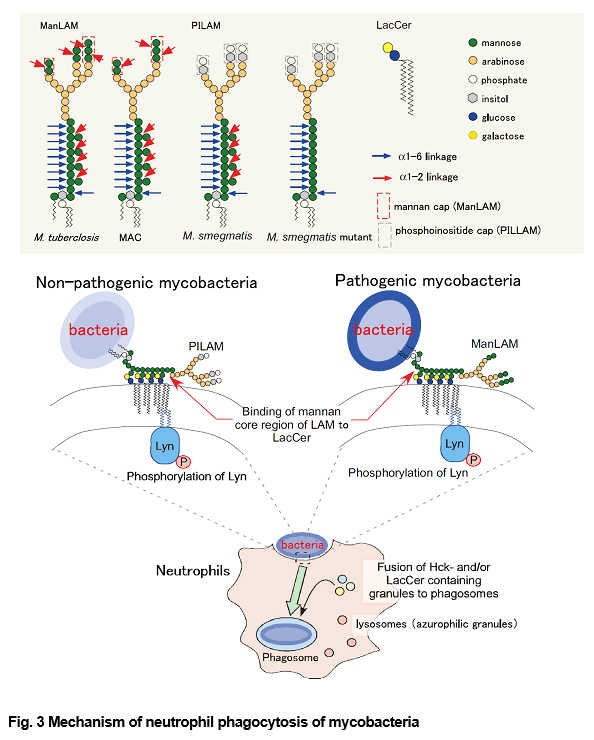
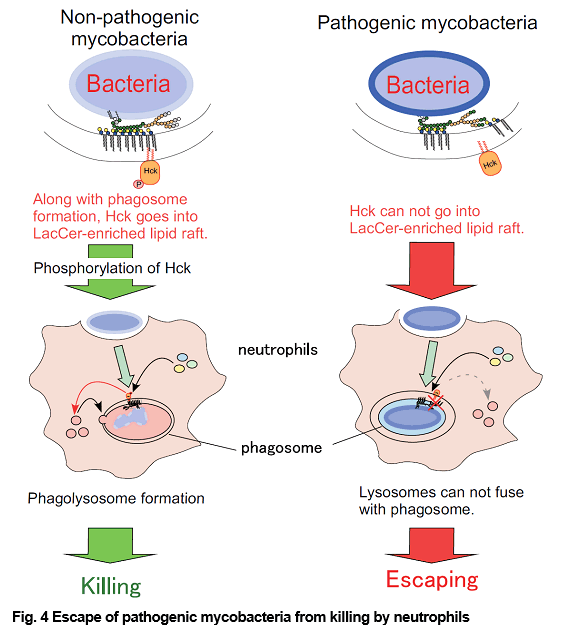
(Sci Signal. Oct 11;9(449):ra101, 2016; Glycobiology. 25: 655-68, 2015; J Leuk. Biol. 83: 728-41, 2008)
Project 3: Roles of phosphatidylglucoside in differentiation and apoptosis of phagocytes and immunity
Phosphatidylglucoside (PtdGlc) is a unique cell surface glycophospholipid originally found in human cord RBCs. PtdGlc has been demonstrated to be associated with cell differentiation. For example, treatment with the recombinant anti-PtdGlc Fab antibody rGL-7 induced HL-60 cell differentiation into neutrophilic cells. Because of the physicochemical property of PtdGlc, this glycolipid forms lipid rafts on plasma membranes. Indeed, PtdGlc was recovered in the Triton X-100-insoluble fractions of HL-60 cells, early erythroblastic leukemia cells and astroglial cells from fetal rat brain by sucrose density-gradient ultracentrifugation. PtdGlc formed different lipid rafts from LacCer-enriched lipid rafts, and was colocalized with Fas on human neutrophils. A series of provocative discoveries led to the hypothesis that neutrophil programmed cell death is the result of an apoptosis-differentiation program, a final stage of transcriptionally regulated PMN maturation or hematopoietic differentiation. Anti-PtdGlc antibody DIM21induced neutrophil apoptosis. DIM21-induced neutrophil apoptosis was mediated by caspases 3, 8, and 9, and inhibited by the neutralizing anti FAS antibody ZB4. These observations clearly suggest that PtdGlc-enriched lipid rafts are involved in Fas-mediated neutrophil apoptosis. Now, we are studying about the molecular mechanisms of PtdGlc-mediated differentiation and immunological functions.
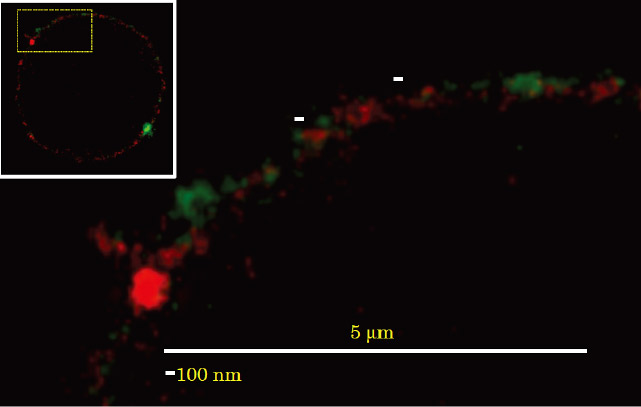
Fig. 5 Colocalization of PtdGlc and LacCer on neutrophils. Neutrophils were stained with Alexa 488-conjugated anti-PtdGlc IgM (green) and Atto425-conjugated anti-LacCer IgM (red). The stained cells were examined with a TCS STED CW confocal microscope equipped with a Plan-Apochromat 100 × oil immersion DIC objective. PtdGlc was not colocalized with LacCer on the plasma membrane of neutrophils.
(Biofactors. 38:275-83, 2012)
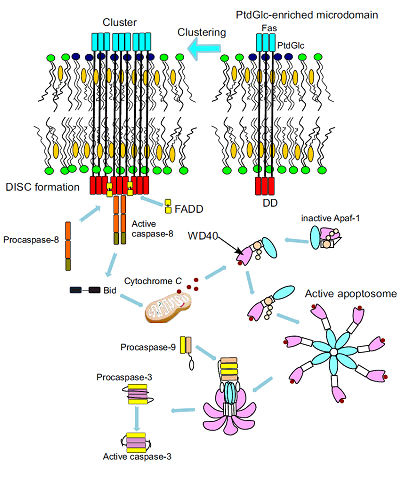
The binding of anti-PtdGlc antibody to PtdGlc induced large cluster formation with Fas, resulting in recruitment of adaptor molecules, such as Fas-associating protein with death domain (FADD) and caspase-8, to form death-inducing signaling complex (DISC). Then procaspase 8 undergoes autocatalytic activation, and subsequently activates capase-9 and caspase-3, resulted in apoptosis of neutrophils. (Inflammation and Regeneration 32: 213-221, 2012)
(BBA (Molecular and Cell Biology of Lipids) 1851:90-7, 2015; Biofactors. 38:275-83, 2012; J Immunol 186: 5323-32, 2011)
Project 4: Role of glycolipid metabolites in immunological reactions of human skin
The epidermis consists of a single layer of proliferating undifferentiated keratinocytes, the stratum basale, and several superficial layers of the stratum spinosum and stratum granulosum (SG), which form the stratum corneum (SC). Ceramide is the main component of SC and is important for the water retention and permeability barrier functions of SC. Ceramides is a major component of SC lipids. CDase hydrolyzed ceramide to yield sphingosine and fatty acids. Sphingosine is phosphorylated by sphingosine kinase to form S1P, which is involved in a wide range of cellular functions, including growth, differentiation, survival, chemotaxis, angiogenesis and embryogenesis, in various types of cells. A neutral CDase from Pseudomonas aeruginosa AN17 (PaCDase) isolated from a patient with atopic dermatitis, and enhanced TNF-α mRNA expression in 3D keratinocytes in the presence of 0.1% Triton X-100, while the increment of TNF-α mRNA level was not observed in cells treated with Triton X-100 alone. Triton X-100 is a detergent that reduces permeability barrier functions, thereby moderately increasing transepidermal water loss and the production of erythema on human skin. TNF-α, a critical cytokine in several dermatological diseases, is secreted by keratinocytes, and shown to be involved in the progression of atopic dermatitis (AD). Among the metabolites of ceramide, only sphingosine and S1P enhanced TNF-α mRNA levels in 3D keratinocytes. S1P is synthesized from sphingosine by sphingosine kinase (SphK) and stimulates 3D keratinocytes through specific receptors. The S1P-induced production of these inflammatory mediators is mediated by S1P receptors in human primary keratinocytes in a 3D culture system. S1P induces TNF-α production and release from 3D keratinocytes via S1P receptors, resulting in TNF-α induction of IL-8 and endothelin-1 productions through NF-κB-mediated signal transduction (Fig. 7). TNF-α is a critical cytokine in psoriatic immunopathology, and the development of an effective strategy is required to counteract its effects. Infliximab, which is used to treat patients with plaque psoriasis, psoriatic arthritis, pustular psoriasis (excluding localized type), and psoriatic erythroderma, downregulates anti-apoptotic proteins in regressing psoriatic skin. Ceramide metabolites, especially S1P, have been demonstrated to be involved in AD. AD is a common pruritic, inflammatory skin disorder (79). Chronic, localized, or even generalized pruritus is the diagnostic hallmark of AD. Histamine H1-receptor blockers are used to treat all types of itch resulting from serious skin diseases, such as AD, as well as from renal and liver diseases. However, they often lack efficacy in chronic itch, a profound clinical problem that decreases quality of life. Nerve density in the epidermis is partly involved in itch sensitization in pruritic skin diseases, such as AD. Endothelin-1 has been shown to elicit itch in humans. The molecular pathways that contribute to the transduction of itch responses to endothelin-1 do not require either PLCβ3 or TRPV1 of neurons, which mediate histamine- and serotonin-induced itch responses, respectively. Thus, keratinocyte-produced S1P may be involved in endothelin-1-mediated pruritus in AD. Now we are studying about the biological functions of glycolipids and their metabolites in skin.
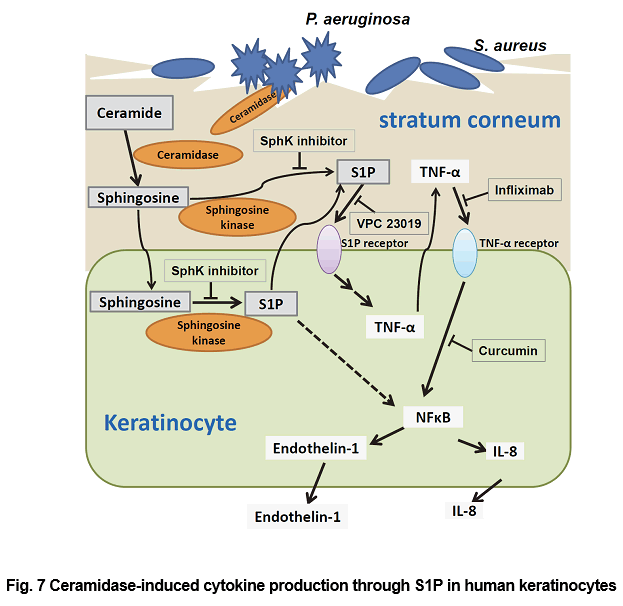
(Mediators Inflamm. 2015:120748, 2015; PLoS One 10.1371/journal.pone.0089402, 2014)
Publication list:
Original article (English only)- Nakayama H, Kurihara H, Morita YS, Kinoshita T, Mauri L, Prinetti A, Sonnino S, Yokoyama N, Ogawa H, Takamori K, Iwabuchi K. Lipoarabinomannan binding to lactosylceramide in lipid rafts is essential for the phagocytosis of mycobacteria by human neutrophils. Sci Signal. Oct 11;9(449):ra101, 2016
- Sueyoshi K, Sumi Y, Inoue Y, Kuroda Y, Ishii K, Nakayama H, Iwabuchi K, Kurishita Y, Shigemitsu H, Hamachi I, Tanaka H. Fluorescence imaging of ATP in neutrophils from patients with sepsis using organelle-localizable fluorescent chemosensors. Ann Intensive Care. Dec;6(1):64, 2016
- Murate M, Abe M, Kasahara K, Iwabuchi K, Umeda M, Kobayashi T. Transbilayer lipid distribution in nano scale. J Cell Sci. 128:1627-3820, 2015
- Chiricozzi E, Ciampa MG, Brasile G, Compostella F, Prinetti A, Nakayama H, Ekyalongo RC, Iwabuchi K, Sonnino S, Mauri L: Direct interaction, instrumental for signaling processes, between LacCer and Lyn in the lipid rafts of neutrophil-like cells. J Lipid Res, 56:129-41, 2015
- Iwabuchi K, Masuda H, Kaga N, Nakayama H, Matsumoto R, Iwahara C, Yoshizaki F, Tamaki Y, Kobayashi T, Hayakawa T, Ishii K, Yanagida M, Ogawa H, Takamori K: Properties and Functions of Lactosylceramide from Mouse Neutrophils. Glycobiology. 25: 655-68, 2015
- Oizumi A, Nakayama H, Okino N, Iwahara C, Kina K, Matsumoto R, Ogawa H, Takamori K, Ito M, Suga Y, Iwabuchi K. Pseudomonas-derived ceramidase induces production of inflammatory mediators from human keratinocytes via sphingosine-1-phosphate. PLoS One 10.1371/journal.pone.0089402, 2014
- Shima S, Kawamura N, Ishikawa T, Masuda H, Iwahara C, Niimi Y, Ueda A, Iwabuchi K, and Mutoh T. Anti-neutral Glycolipids Antibodies In Encephalomyeloradiculoneuropathy. Neurology, 82(2):114-8. 2014
- Nozawa K, Fujishiro M, Kawasaki M, Yamaguchi A, Ikeda K, Morimoto S, Iwabuchi K, Yanagida M, Ichinose S, Morioka M, Ogawa H, Takamori K, Takasaki Y, Sekigawa I. Inhibition of connective tissue growth factor ameliorates rheumatoid arthritis in a murine model. Arthritis Rheum. 65(6):1477-86, 2013
- Watanabe K. Iwahara C. Nakayama H. Iwabuchi K, Matsukawa T, Yokoyama K, Yamaguchi K, Kamiyama Y and Inada E. Sevoflurane Suppresses TNF-α-Induced Inflammatory Responses in Small Airway Epithelial Cells under Anoxia/Reoxygenation Conditions. Bri. J Anaesth. 110(4): 637-45, 2013
- Fu L, Kitamura T, Iwabuchi K, Ichinose S, Yanagida M, Ogawa H, Watanabe S, Maruyama T, Suyama M, Takamori K. Interplay of neuropilin-1 and semaphorin 3A after partial hepatectomy in rats. World J Gastroentero 18: 5034-41, 2012
- Watanabe S, Higashi H, Ogawa H, Takamori K, Iwabuchi K. Involvement of ganglioside GT1b in glutamate release from neuroblastoma cells. Neurosci Lett. 31;517:140-3.2012
- Tabe Y, Jin L, Iwabuchi K, Wang R, Ichikawa N, Miida T, Cortes J, Andreeff M, Konopleva M. Role of stromal microenvironment in nonpharmacological resistance of CML to imatinib through Lyn/CXCR4 interactions in lipid rafts. Leukemia. 26:883-92, 2012
- Fujishiro M, Nozawa K, Kawasaki M, Yamaguchi A, Iwabuchi K, Yanagida M, Suzuki F, Miyazawa K, Fukui H, Kaneko K, Ogawa H, Takamori K, Takasaki Y, Sekigawa I. Regenerating gene (REG) 1 alpha promotes pannus progression in patients with rheumatoid arthritis. Mod Rheumatol. 22: 228-37, 2012
- Nishioka J, Iwahara C, Kawasaki M, Yoshizaki F, Nakayama H, Takamori K, Ogawa H, Iwabuchi K. Di-(2-ethylhexyl) phthalate induces production of inflammatory molecules in human macrophages. Inflamm Res. 61: 69-78, 2012
- Kina K, Masuda H, Nakayama H, Nagatsuka Y, Nabetani T, Hirabayashi Y, Takahashi Y, Shimada K, Daida H, Ogawa H, Takamori K, Iwabuchi K: The novel neutrophil differentiation marker phosphatidylglucoside mediates neutrophil apoptosis. J Immunol 186: 5323-32, 2011.
- Kiyanagi T, Iwabuchi K, Shimada K, Hirose K, Miyazaki T, Sumiyoshi K, Iwahara C, Nakayama H, Masuda H, Mokuno H, Sato S, Daida H: Involvement of Cholesterol-enriched Microdomains in Class A Scavenger Receptor-mediated Responses in Human MACrophages. Atherosclerosis 215:60-9, 2011.
- Hirose K, Iwabuchi K, Shimada K, Kiyanagi T, Iwahara C, Nakayama H, Daida H: Different responses to oxidized low-density lipoproteins in human polarized macrophages. Lipids Health Dis. Jan 4; 10 (1): 1, 2011.
- Nozawa K, Fujishiro M, Kawasaki M, Kaneko H, Iwabuchi K, Yanagida M, Suzuki F, Miyazawa K, Takasaki Y, Ogawa H, Takamori K, Sekigawa I.Connective tissue growth factor promotes articular damage by increased osteoclastogenesis in patients with rheumatoid arthritis. Arthritis Res Ther. 11:R174, 2009
- Ichikawa N, Iwabuchi K, Kurihara H, Ishii K, Kobayashi T, Sasaki T, Hattori N, Mizuno Y, Hozumi K, Yamada Y, Arikawa-Hirasawa E. Binding of laminin-1 to monosialoganglioside GM1 in lipid rafts is crucial for neurite outgrowth. J Cell Sci. 122: 289-99. 2009
- Nakayama H, Yoshizaki F, Prinetti A, Sonnino S, Mauri L, Takamori K, Ogawa H, Iwabuchi K.: Lyn-coupled LacCer-enriched lipid rafts are required for CD11b/CD18-mediated neutrophil phagocytosis of non-opsonized microorganisms. J Leuk. Biol. 83: 728-41, 2008
- Iwabuchi K, Prinetti A, SonninovS, MaurivL, Kobayashi T, Ishii K, Kaga N, Murayama K, Kurihara H, Nakayama H, Yoshizaki F, Takamori K, Ogawa H, Nagaoka I.: Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconjugate J. 25: 357-74, 2008
- Yanagida M, Nakayama H, Yoshizaki F, Fujimura T, Takamori K, Ogawa H, Iwabuchi K.: Proteomic analysis of plasma-membrane lipid rafts of HL-60 cells., Proteomics, 7: 2398-2409, 2007
- Kurokawa A, Azuma K, Mita T, Toyofuku Y, Fujitani Y, Hirose T, Iwabuchi K, Ogawa H, Takeda S, Kawamori R, Watada H. 2-Methoxyestradiol reduces monocyte adhesion to aortic endothelial cells in ovariectomized rats. Endocr J. 54: 1027-31, 2007
- Sato T, Iwabuchi1 K, Nagaoka I, Adachi Y, Ohno N, Tamura H, Seyama K, Fukuchi F, Nakayama H, Yoshizaki F, Takamori K, and Ogawa H.: Induction of human neutrophil chemotaxis by Candida albicans-derived β-1,6 long glycoside side chains-branched β-glucan. J Leukocyte. Biol. 80(1):204-11, 2006
- Miyaji A, Ohwada A, Iwabuchi K, Ogawa H, Fukuchi Y.: Decreased salivary vascular endothelial growth factor in elderly patients with pneumonia during the course of recovery. Geriatrics and Gerontology International, 6 182-185, 2006
- Kashiwakura1 Y, Tamayose K, Iwabuchi K, Hirai Y, ShimadaT, MatsumotoK, Nakamura T, Watanabe M, Oshimi K, Daida H.: HGF receptor is a coreceptor for adeno-associated virus type 2 (AAV2) infection. J. Virology 79: 609-14, 2005
- Kaga N, Kazuno S, Taka H, Iwabuchi K, and Murayama K.: Isolation and characterization of molecular species of lactosylceramides derived from HL-60 and porcine blood cells using liquid chromatography/electrospray ion trap mass spectrometry. Anal. Biochem. 337, 316-24, 2005
- Takaya N, Katoh Y, Iwabuchi K, Hayashi I, Konishi H, Itoh S, Okumura K, Ra C, Nagaoka I, Daida H. Platelets activated by collagen through t5.he immunoreceptor tyrosine-based activation motif in the Fc receptor gamma-chain play a pivotal role in the development of myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 39(6): 856-64, 2005
- Sato S, Ishii K, Makino A, Iwabuchi K, Yamaji-Hasegawa A, Senoh Y, Nagaoka I, Sakuraba H, Kobayashi T. Distribution and transport of cholesterol-rich membrane domains monitored by a membrane-impermeant fluorescent poly (ethylene glycol)-derivatized cholesterol. J.Biol.Chem. 279, 23790-6, 2004
- Hua J., Suguro S., Iwabuchi K., Tsutsumi-Ishii Y., Sakamoto K., Nagaoka I.: Glucosamine, a naturally ccurring amino monosaccharide suppresses the ADP-mediated platelet activation in humans. Inflammation Res. 53, 680-8, 2004
- Iwabuchi K, Nakayama H, Oizumi A, Suga Y, Ogawa H, Takamori K. Role of Ceramide from Glycosphingolipids and Its Metabolites in Immunological and Inflammatory Responses in Humans. Mediators Inflamm. 2015:120748, 2015
- Ekyalongo R, Nakayama H, Kina K, Kaga N, Iwabuchi K. Organization and functions of glycolipid-enriched microdomains in phagocytes. BBA (Molecular and Cell Biology of Lipids) 1851:90-7, 2015
- Iwabuchi K. Involvement of glycosphingolipid-enriched lipid rafts in inflammatory responses. Front Biosci (Landmark Ed), 20:325-34, 2015
- Nakayama H, Ogawa H, Takamori K, Iwabuchi K. GSL-enriched membrane microdomains in innate immune responses. Arch. Immuno. et Therap. Exp. 61(3):217-28, 2013
- Kina K, Masuda H, Nakayama H, Iwahara C, Nagatsuka Y, Hirabayashi Y, Ogawa H, Takamori K, and Iwabuchi K: The novel neutrophil differentiation marker phosphatidylglucoside is involved in Fas-dependent apoptosis. Inflammation and Regeneration 32: 213-221, 2012
- Iwabuchi K, Nakayama H, Masuda H, Kina K, Ogawa H, Takamori K. Membrane microdomains in immunity: Glycosphingolipid-enriched domain-mediated innate immune responses. Biofactors. 38(4):275-83, 2012.
- Iwabuchi K, Nakayama H, Iwahara C, Takamori K.: Significance of glycosphingolipid fatty acid chain length on membrane microdomain-mediated signal transduction. FEBS Lett. 584(9):1642-52, 2010
- Sonnino S, Prinetti A, Nakayama H, Yangida M, Ogawa H, Iwabuchi K. Role of very long fatty acid-containing glycosphingolipids in membrane organization and cell signaling: the model of lactosylceramide in neutrophils. Glycoconj J. 26(6): 615-21, 2009
- Yoshizaki F, Nakayama H, Iwahara C, Takamori K, Ogawa H, Iwabuchi K.: Role of glycosphingolipid-enriched microdomains in innate immunity: Microdomain- dependent phagocytic cell functions. Biochim. Biophys. Acta 1780: 383-392, 2008
- Nakayama H, Iwahara C, Ogawa H, Takamori K, Iwabuchi K. Lactosylceramide is a Pattern Recognition Receptor that Forms Lyn-Coupled Membrane Microdomains on Neutrophils Immun., Endoc. & Metab. Agents in Med. 8(4): 327-335, 2008
- M Yanagida, K Iwabuchi. Proteomic analysis of lactosylceramide-enriched membrane microdomains . Trends in Glycosci. Glycotech. 20: 1-15, 2008
- Iwabuchi K.: Structure and functions of glycosignaling domain. Trends in Glycosci. Glycotech. 17, 1-14, 2005
- Nakayama H, Iwabuchi K: Glycosphingolipid-Receptor Interactions in the Innate Immune Responses. Glycoscience: Biology and Medicine, Part VII, pp699-705, 2014.
- Hirose K, Kiyanagi T, Shimada K, Daida H, Iwabuchi K:Macrophages: Life Cycle, Functions and Participation in the Pathogenesis of Atherosclerosis in Handbook of Macrophages: Life Cycle, Functions and Diseases. Nova Science Publishers pp423-433, 2012


