- Home
- Research
Research
Background of research and research objectives
The proper turnover of cellular proteins is essential for cellular homeostasis, and defects in this process lead to various serious diseases. Macroautophagy (hereafter referred to as autophagy) is a cellular degradation system in which cytoplasmic components, including organelles, are sequestered by double-membrane structures called autophagosomes and the sequestered materials are degraded by lysosomal hydrolases (Figure 1). The resulting amino acids, glucose, and free fatty acids are utilized in macromolecule synthesis and as an energy source .
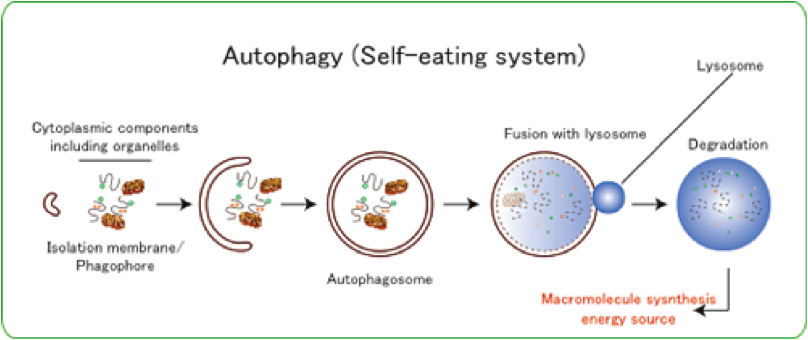
Figure 1) Schematic diagram of autophagy. The degradation system, including membrane dynamics, is induced in response to cellular emergencies, e.g., nutrient deprivation. The isolation membranes/phagophores engulf cytoplasmic constituents, including organelles, and the resulting autophagosomes fuse with lysosomes, resulting in the complete degradation of the sequestered cytoplasmic components by lysosomal hydrolases.
Although autophagy has generally been considered non-selective, recent studies have shed light on another indispensable role for basal autophagy in cellular homeostasis, which is mediated by the selective degradation of a specific substrate(s), e.g., ubiquitin-positive large aggregate structures and damaged mitochondria (Figure 2). Therefore, the loss of autophagy causes the build-up of inclusion bodies and deformed organelles, resulting in life-threatening diseases, including neurodegeneration and cancer, even in nutrient-rich conditions. In this project, we aim to clarify the pathophysiological roles of selective autophagy as well as its molecular mechanisms.
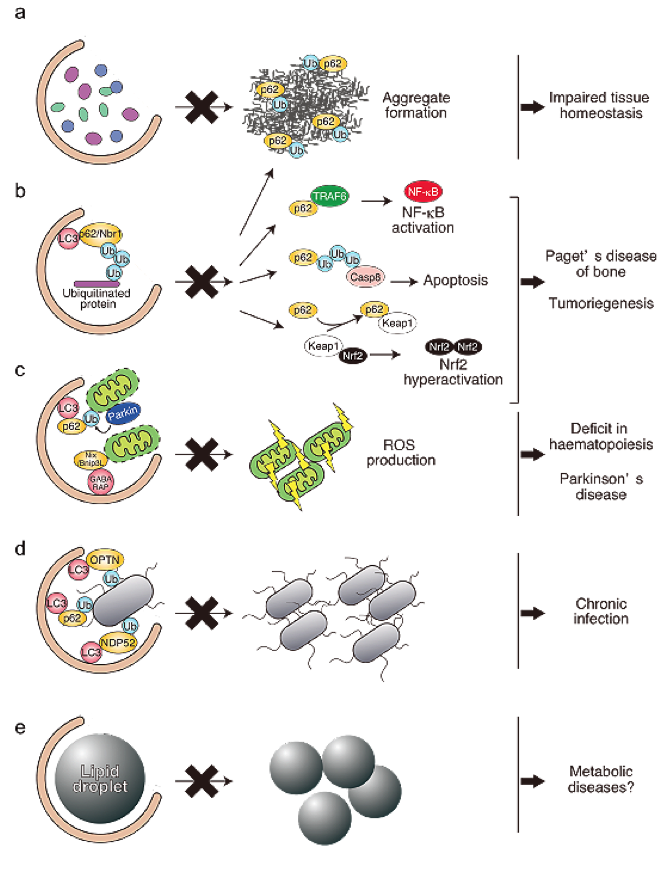
Figure 2) Physiological and pathological relevance of selective types of autophagy. (a) Autophagy operates constitutively at low levels, even under nutrient-rich conditions, and mediates the global turnover of cytoplasmic material. Defects in autophagy are accompanied by the disruption of tissue homeostasis, resulting in life-threatening diseases.(b) The LC3-binding proteins p62 and Nbr1 have a UBA domain through which they can mediate the sequestration of ubiquitinated cargos into autophagosomes. Meanwhile, p62 itself is also a specific substrate for autophagy. Defective autophagy is usually accompanied by the extensive accumulation of p62, which enhances its function as a scaffold protein in several signaling pathways, e.g., NF-κB signaling, apoptosis, and Nrf2 activation. Such abnormalities might be involved in tumorigenesis and Paget disease of bone. (c) Nix/Bnip3 localizes to mitochondria and leads to their depolarization, which is indispensable for mitophagy during erythroid differentiation. Loss of Nix/Bnip3 is accompanied by erythroid maturation arrest, leading to severe anemia. In response to the loss of mitochondrial membrane potential, Parkin translocates to damaged mitochondria in a PINK1-dependent manner, and ubiquitinates the outer membrane proteins, thereby inducing mitophagy. Parkinson disease-related mutations of Parkin and PINK1 are unable to induce mitophagy, suggesting that the remaining damaged mitochondria, due to inadequate mitophagy, have a role in the pathogenesis of Parkinson disease. (d) Invading bacteria in the cytosol and/or ruptured endosomal membranes are ubiquitinated, and autophagic receptors, including p62, NDP52, and optineurin, mediate the autophagic sequestration of the microbes to restrict their growth.(e) Cellular lipid droplets are degraded by autophagy, probably in a selective manner. This type of selective autophagy (lipophagy) supplies free-fatty acids that are utilized for β-oxidation. Defective lipophagy is known to cause the accumulation of lipid droplets in hepatocytes and the reduced production of Agouti-related peptides (AgRP) in AgRP-expressing neurons.
Research characteristics
On the basis of our strong expertise in protein biochemistry and mouse genetic studies, we relentlessly pursue a revolutionary approach to the understanding of the role of selective autophagy in the suppression of tissue degeneration and other human diseases such as cancer and neurodegeneration.