Laboratory of Molecular and Biochemical Research
Research
We are now working on the following projects.
Stem cells possess two main attributes, namely, a self-renewal capacity and multipotency. Self-renewal refers to the ability of a cell to replicate itself without differentiating or losing multipotency. Multipotency is the ability to differentiate into more than one cell lineage. Stem cells repeat self-renewal and differentiation to produce tissues and organ in our body. Interestingly, stem-like cells exist also in the tumor tissues, and are called "cancer stem cells". It is thought that cancer stem cells produce tumor tissues and are responsible for cancer recurrence. However, the nature of cancer stem cells is still unknown.
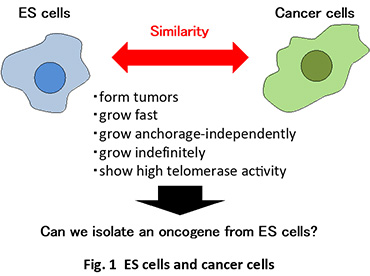
We are now focusing our attention on the similarity between embryonic stem (ES) cells and cancer stem cells (Fig. 1). Both cells show similar properties, including tumor formation ability, high telomerase activity and indefinite growth. These stem cells display similarities also at a molecular level(Koide, 2014). For example, STAT3 and Myc, which are involved in tumor growth, play an important role in ES cell self-renewal, while two critical factors for ES cell self-renewal, Oct3/4 and Sox2, participate in tumor growth. We have demonstrated that β-catenin and Etv4/5, which are implicated in tumor development and growth, regulate the self-renewal in ES cells(Takao et al., 2007; Kelly et al., 2011; Akagi et al., 2015).
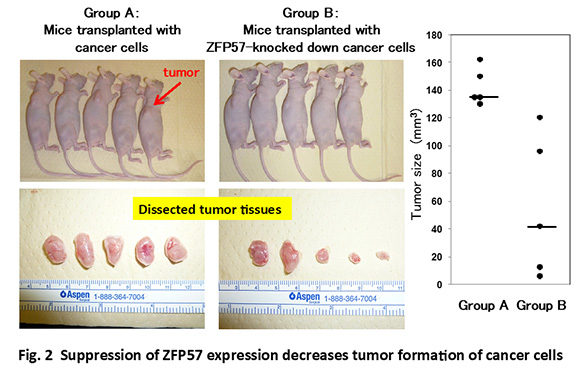
These similarities, as well as the genetic stability and the little genetic variation of ES cells, encouraged us to isolate a novel oncogene(s) from genes that are expressed in self-renewing ES cells. We have succeeded to identify an ES-specific transcription factor Zfp57 as a novel oncogene (Tada et al., 2015)(Fig. 2). Furthermore, we found that Zfp57 is involved in the metastasis of colorectal cancer(Shoji et al., 2019). We also found that an ES-specific transcription factor Nanog positively regulates Zfp57 expression (Yamaguchi et al., 2014; Shoji et al., 2019).
We are currently examining the molecular mechanism of Zfp57-induced tumorigenesis, as well as searching for another ES-specific oncogene.
To utilize ES and iPS cells for regenerative medicine, we need to expand these cells efficiently to obtain sufficient amount of cells for transplantation. Previous studies demonstrated that ACTH, Shh and activin promote ES cell growth.
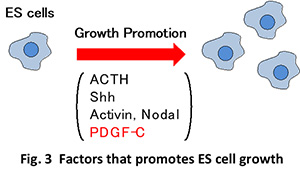
We are searching for such ES growth factors by taking the advantage of the similarity between ES and cancer stem cells. More specifically, we are exploring the possibility that tumor growth-promoting factors may enhance ES cell growth. In fact, we recently found that PDGF-C can promote the growth of ES cells(Kinjo et al., 2018)(Fig. 3).
We are currently examining the molecular mechanism of PDGF-C-stimulated proliferation, as well as searching for another growth factor for ES cells.
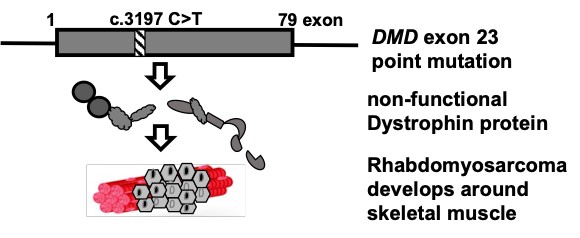
Dystrophin gene (DMD) is a huge gene responsible for Duchenne muscular dystrophy (DMD), an incurable inherited progressive skeletal muscle wasting disease. Recently, the gene is recognized as a tumor suppressor and an anti-metastatic agent because, DMD intragenic mutations were detected in tumors (sarcomas) that originate from skeletal muscle precursor cells. We have two projects that will be focused on deciphering the dystrophin gene or transcript.
The project is to investigate the hydrophilic metabolomic profiles in tumors from mouse (mdx) models of Duchenne muscular dystrophy (DMD). The mdx mouse has been reported to spontaneously produce tumor (Fig. 1). We also observed that DMD patients spontaneously develop various tumors (Niba et al., 2020). However, the mechanism is not clear. Additionally, the metabolites that contribute to tumor growth in a dystrophic system are not clearly established. We constructed six mdx mouse and monitored them for the spontaneous development of different tumors after about 17-22 weeks. Analysis of the metabolomic profile in one of them showed difference to skeletal muscle (Fig. 2). This study aims to identify hydrophilic metabolites in these tumors that may contribute to their spontaneous growth, and invasiveness through autophagy and/or epithelial-mesenchymal transition for novel cancer therapies.
We are now studying the metabolites that contribute to spontaneous tumor growth.
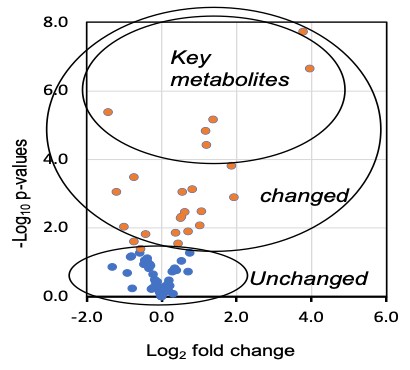
We are now trying to understand the link of key metabolites to tumor growth and invasion.
Rhabdomyosarcoma (RMS) is the most common pediatric cancer in the world arising from skeletal muscle progenitors. Malfunction of the Duchenne muscular dystrophy-related gene, dystrophin was implicated in the growth and metastasis of tumor in RMS and hence, it was validated to be a tumor suppressor and a likely anti-metastatic agent in RMS. Here, we aim to investigate the dystrophin gene pre-mRNA splicing (intron retention: IR) for Targeted therapy development, because IR leads to malfunctional dystrophin and hence might decrease factors and agents that promote tumor suppression in RMS (Niba et al., 2017, Kawaguchi and Niba et al., 2018) (Fig. 3).
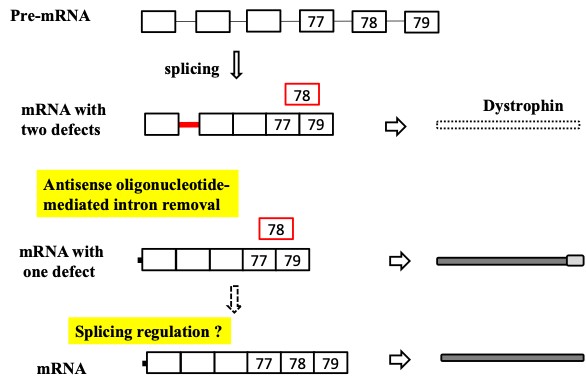
What is the mechanism by which the effect of antisense restores dystrophin function?
Are there any signaling molecules involved such as Ca2+?
- Akagi et al. (2015) ETS-related transcription factors ETV4 and ETV5 are involved in proliferation and induction of differentiation-associated genes in ES cells. J. Biol. Chem. 290, 22460-22473.
- Kawaguchi and Niba et al. (2018) Detection of dystrophin Dp71 in human skeletal muscle using an automated capillary Western assay system. Int. J. Mol. Sci. 19, 1546.
- Kelly et al. (2011) β-catenin enhances Oct-4 activity and reinforces pluripotency through a TCF-independent mechanism. Cell Stem Cell 8, 214-227.
- Kinjo et al. (2018) Platelet-derived growth factor-C functions as a growth factor in mouse embryonic stem cells and human fibrosarcoma cells. Cell. Mol. Biol. Lett. 23, 8.
- Koide (2014) Embryonic stem cells and oncogenes. in Pluripotent Stem Cell Biology - Advances in Mechanisms, Methods and Models (Craig S. Atwood & Sivan Vadakkadath Meethal eds.) pp 41-61, InTech.
- Niba et al. (2017) DMD transcripts in CRL-2061 rhabdomyosarcoma cells show high levels of intron retention by intron-specific PCR amplification. Cancer Cell Int. 17, 58.
- Niba et al. (2020) Pilocytic astrocytoma tissue from a 25-year-old patient with Duchenne Muscular Dystrophy expresses the DMD Transcript but not protein. Clin. Res. Pediatr. 3, 1-9.
- Shoji et al. (2019) The ES cell-specific transcription factor ZFP57 promotes liver metastasis of colorectal cancer. J. Surg. Res. 237, 22-29.
- Tada et al. (2015) The stem cell transcription factor ZFP57 induces IGF2 expression to promote anchorage-independent growth in cancer cells. Oncogene 34, 752-760.
- Takao et al. (2007) β-Catenin up-regulates Nanog expression through interaction with Oct-3/4 in embryonic stem cells. Biochem. Biophys. Res. Commun. 353, 699-705.
- Yamaguchi et al. (2014) Nanog positively regulates Zfp57 expression in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 453, 817–820.


