Department of Pathology and Oncology【Department of Molecular Pathogenesis】
RESEARCH
- Molecular mechanisms of malignancy promotion by cancer-associated fibroblasts
- Interaction between cancer cells and stroma in the tumor microenvironment
- Mechanisms of drug resistance and development of novel therapies
Molecular mechanisms of malignancy promotion by cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) refer to fibroblasts that exist in the stroma adjacent to cancer cells within tumors. CAFs are not merely fibroblasts located in the vicinity of cancer cells, but have acquired properties distinct from normal fibroblasts. They play various roles in promoting cancer malignancy, including tumor growth, tumor angiogenesis, metabolic reprogramming, invasion and metastasis, and resistance to treatment. In addition, it has been reported that CAFs sometimes work in a direction that suppresses cancer. The importance of CAFs has been demonstrated in various types of cancer, including breast, colorectal, pancreatic, and esophageal cancers. Therefore, gaining a deeper understanding of CAFs will directly contribute to progressing our comprehension of cancer, making it an extremely important research topic. However, research on CAFs lags behind that of cancer cells. One of the reasons why CAF research is challenging is their diversity and heterogeneity and the absence of clear marker proteins to define and distinguish CAFs from other cell types. Just as cancer characteristics and conditions vary from patient to patient, it is natural that the CAFs exhibit diversity and heterogeneity. We believe that research aimed at understanding CAFs and targeting them for treatment requires both meticulous investigation of the details of the complexity and studies that capture the whole research landscape with an overall, bird's-eye view. This approach involves carefully examining each aspect of the complexity while simultaneously advancing research that grasps the big picture.
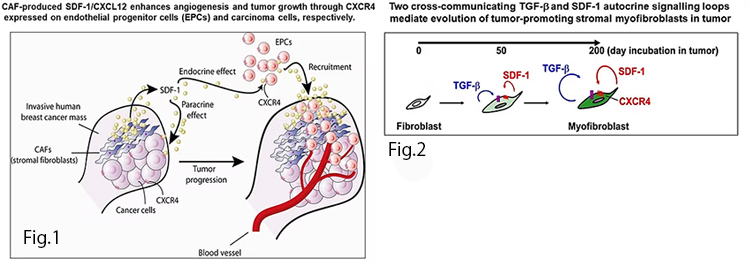
Our research group has been studying the role of CAFs at the molecular, cellular, and individual levels, based upon a mile-stone study that demonstrated that stromal cell-derived factor 1 (SDF-1/CXCL12) secreted from breast cancer CAFs acts through CXCR4 receptors displayed on endothelial progenitor cells (EPCs) and breast cancer cells to promote breast cancer growth and progression. CAF-produced CXCL12 contributes to not only promotion of angiogenesis by recruiting EPCs into the tumor mass in an endocrine fashion, but also acting paracrinely on nearby cancer cells to promote their proliferation (Fig. 1; Orimo et al, Cell, 2005; Orimo and Weinberg, Cell Cycle, 2006). Using CAFs generated from human mammary fibroblasts, we then demonstrated that the acquisition of CXCL12 and TGF-β signaling through an autocrine mechanism plays a critical role in inducing and maintaining both activated myofibroblast functions and cancer-promoting capabilities (Fig. 2; Kojima et al, PNAS, 2010). Further, we reported that CD26 expression is suppressed by CXCL12 and TGF-β autocrine signaling, which could serve as a poor prognostic marker (Mezawa et al, Cancer Med., 2019).
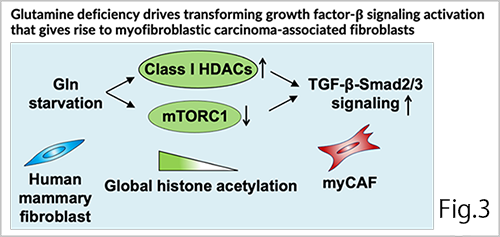
To elucidate the detailed mechanisms underlying the conversion of normal fibroblasts into cancer-promoting CAFs, we focused on epigenetic regulation, transcription factors, and extracellular amino acid concentrations. In a breast cancer model, we proposed that glutamine depletion in the tumor microenvironment induces the emergence of myofibroblastic CAFs by activating TGF-β signaling through the activation of class I histone deacetylases and the suppression of mammalian target of rapamycin complex 1 (mTORC1) (Fig. 3; Mezawa et al, Cancer Sci., 2023). Additionally, we reported that transcription factors, such as runt-related transcription factor 3 (RUNX3) and lymphoid enhancer-binding factor 1 (LEF1) are involved in the tumor-promoting ability of breast CAFs (Koyama et al, Cancer Med., 2023; Okazaki et al, Cancer Med., 2025).
Understanding whether cancer invasion and metastasis are primarily driven by single cancer cells that have undergone complete epithelial-mesenchymal transition (EMT) or by clusters of cancer cells with adhesive properties through hybrid EMT, also known as partial EMT, is a crucial issue in comprehending the process of cancer progression.
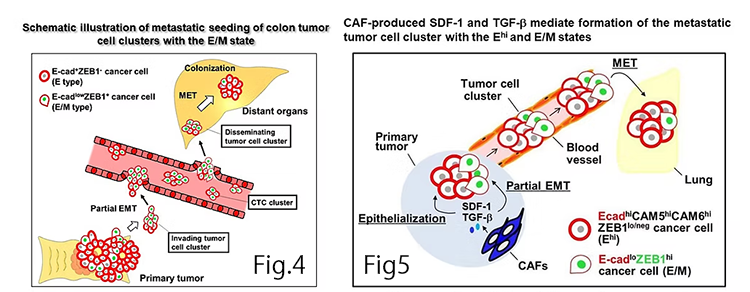
We successfully established patient-derived tumor xenografts (PDX) by transplanting surgically resected human colorectal cancer into immunodeficient mice, and observed spontaneous metastasis of colorectal cancer cells to the liver and lungs. We then conducted immunohistochemical examination of these PDX and patient cancer samples, and proposed a metastasis model (Fig. 4; Mizukoshi et al, Int. J. Cancer, 2020). In the primary tumor site, two types of colorectal cancer cells are present: those with epithelial characteristics expressing E-cadherin (E type) and those exhibiting both epithelial and mesenchymal traits, co-expressing E-cadherin and the transcription factor Zinc Finger E-Box Binding Homeobox 1 (ZEB1) (E/M type) via hybrid EMT. These colorectal cancer cells form clusters, locally invade surrounding tissues, and contribute to metastasis formation in distant organs. Then, metastasized E/M-type cancer cells promote metastatic colony formation by reverting to an epithelial phenotype presumably through mesenchymal-epithelial transition (MET), thus contributing to metastasis formation in the distant organs.
There was no established theory on how CAFs promote cancer invasion and metastasis. Therefore, we conducted research and proposed that CAFs induce the formation of tumor cell clusters through partial/hybrid EMT, resulting in promotion of invasion and metastasis (Fig. 5; Matsumura et al, Life Sci. Alliance, 2019). When human breast cancer cells and CAFs were co-transplanted into immunodeficient mice to form xenograft tumors, the extracted cancer cells exhibited two distinct phenotypes. The first type, referred to as Ehi (E-cadherinhi/ZEB1lo/neg), displayed epithelial characteristics induced by CAF-derived CXCL12 and TGF-β. The second type, referred to as E/M (E-cadherinlo/ZEB1hi), showed hybrid EMT properties, being positive for both E-cadherin and ZEB1. We demonstrated that these two types of cancer cells acquire resistance to cell death and enhanced invasive and metastatic abilities by forming clusters. Furthermore, E/M-type cancer cells that metastasized to the lungs promoted the formation of metastatic colonies by reverting to an epithelial phenotype via MET.
Currently, our laboratory is conducting research to further understand the importance of CAFs in breast cancer, colorectal cancer, pancreatic cancer, and esophageal cancer. We are investigating this at the molecular, cellular, and individual levels. Furthermore, these research projects are being conducted in collaboration with the clinical departments of Juntendo University and Juntendo University Hospital (Nakamura et al, Jpn. J. Clin. Oncol.,2024; Okazaki et al, Cancer Med., 2025). We are advancing our research not only through wet lab experiments but also by utilizing large-scale analysis data available in public databases. We are employing dry analysis methods, including the reanalysis of single-cell RNA-sequencing data, and are currently obtaining interesting insights in several research projects.
Interaction between cancer cells and stroma in the tumor microenvironment
As mentioned above, we have so far focused our research on CAFs among stromal cells. In our ongoing studies, we are conducting comprehensive research not only on CAFs but also on other non-cancerous cells and stromal components within tumors, including immune cells that are deeply involved in cancer progression. Our research aims to understand the complex interactions between these elements and cancer cells. Additionally, we are investigating how these interactions lead to critical phenomena, such as invasion and metastasis, which are the primary causes of cancer-related deaths.
Mechanisms of drug resistance acquisition and development of novel therapies
We are not only developing research to understand cancer, but also advancing research projects aimed at improving current treatments and developing new therapeutic approaches based on the knowledge gained. Additionally, leveraging the specialized research expertise of our faculty members, we are also pursuing projects that incorporate biological issues closely related to cancer, such as mitochondria, primary cilia, and cell polarity.



